When functional improvement with gabapentinoids isn’t enough
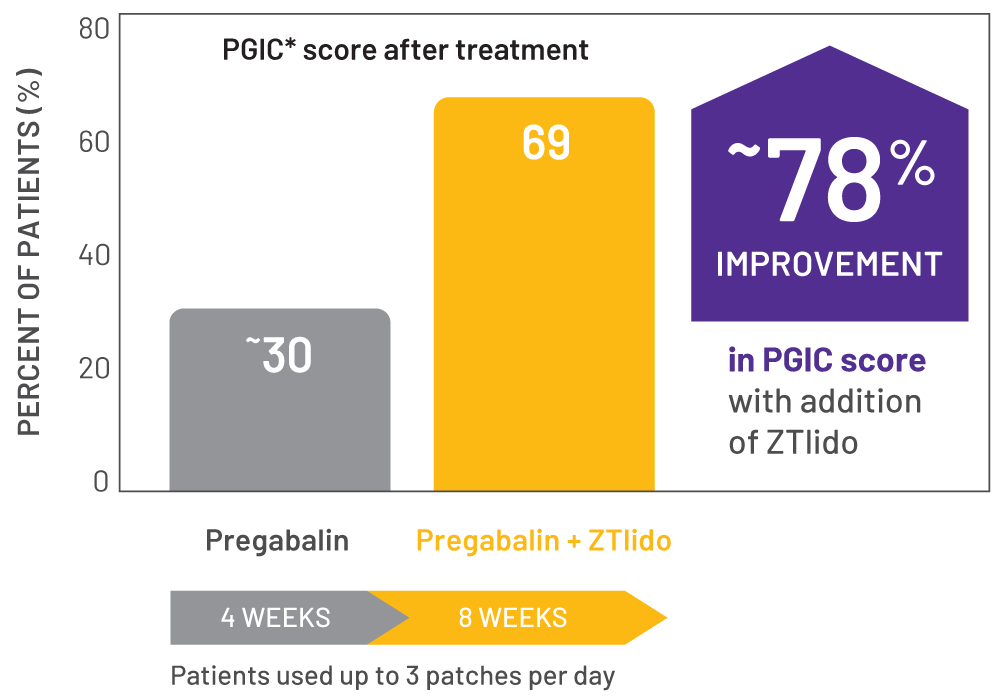
Adding ZTlido enhanced quality of life1,12


CPSI=Chronic Pain Sleep Inventory; PHN=post-herpetic neuralgia; QoL=quality of life; SF-36=Short-Form 36; SF-MPQ=Short-Form McGill Pain Questionnaire.
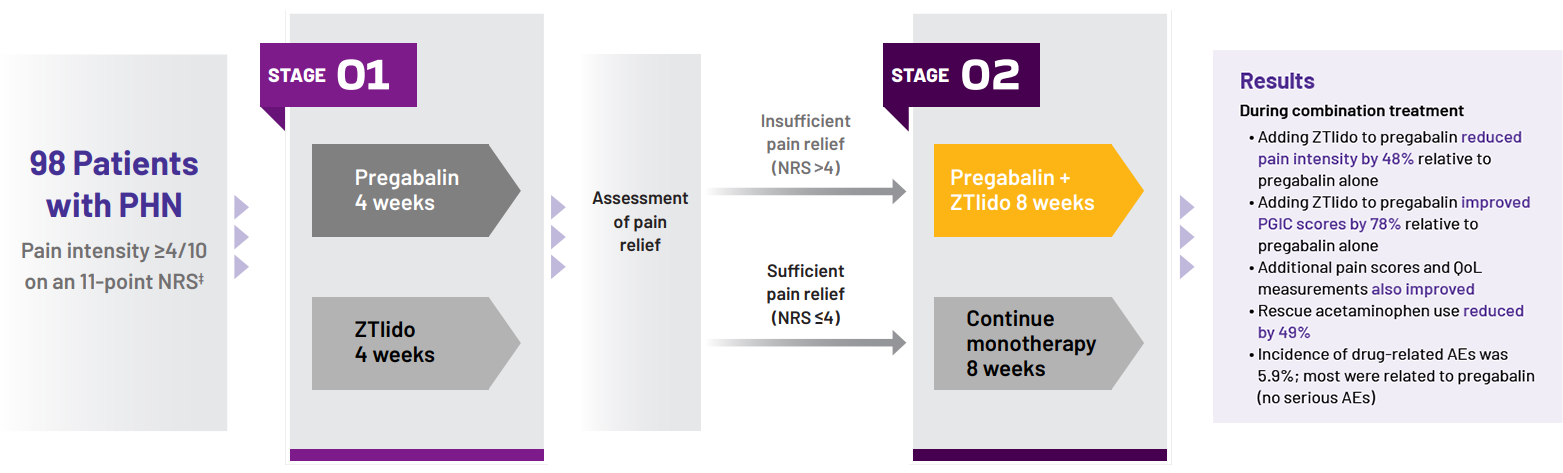
Study design13
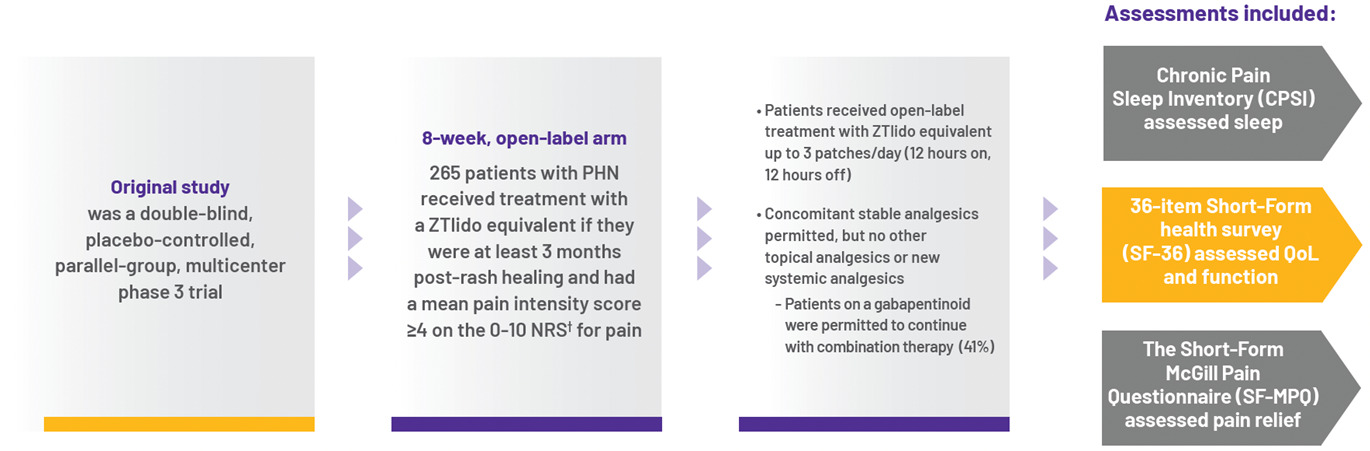
Additional study results:
After 8 weeks of ZTlido treatment, patients experienced:
- Improved sleep parameters (CPSI) and perception of patients' QoL (SF-36)
- Reduced pain burden as assessed by SF-MPQ:
- Approximately 50% of patients achieved at least `moderate` pain relief
†11-point NRS (0=no pain to 10=pain as bad as you can imagine).
CPSI=Chronic Pain Sleep Inventory; PHN=post-herpetic neuralgia; QoL=quality of life; SF-36=Short-Form 36; SF-MPQ=Short-Form McGill Pain Questionnaire.
†ZTlido equivalent. "ZTlido equivalent" connotes that study was performed using bioequivalent lidocaine 5% patch.
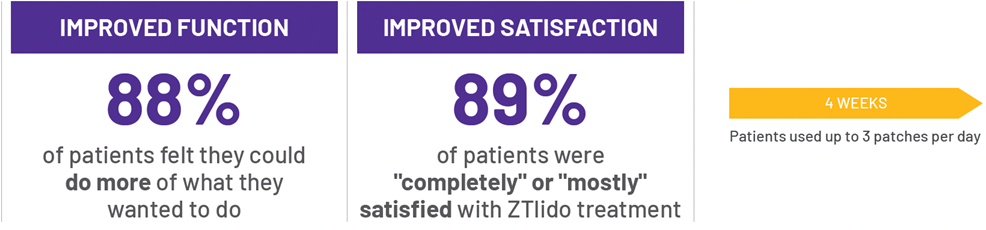
‡Sleep measured by CPSI (usually/always); function measured by SF-36.