Titration to an effective dose is often unattainable3

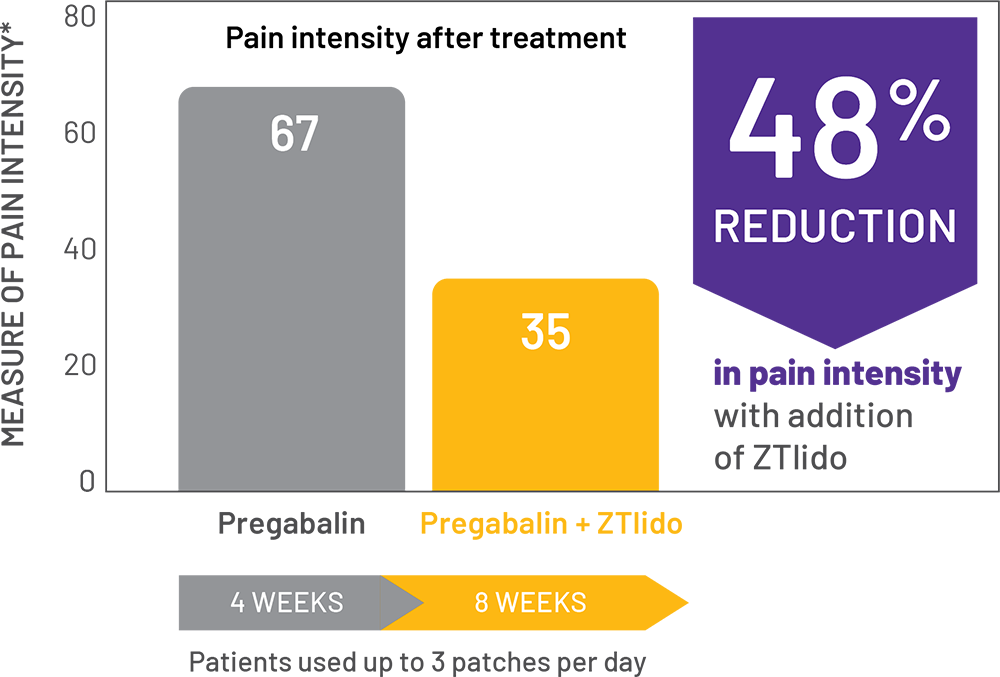
adaptive, randomized, open-label study (N=98) in patients with PHN; chart shows patients treated with pregabalin alone, then in combination with a ZTlido equivalent.1†
Study design1
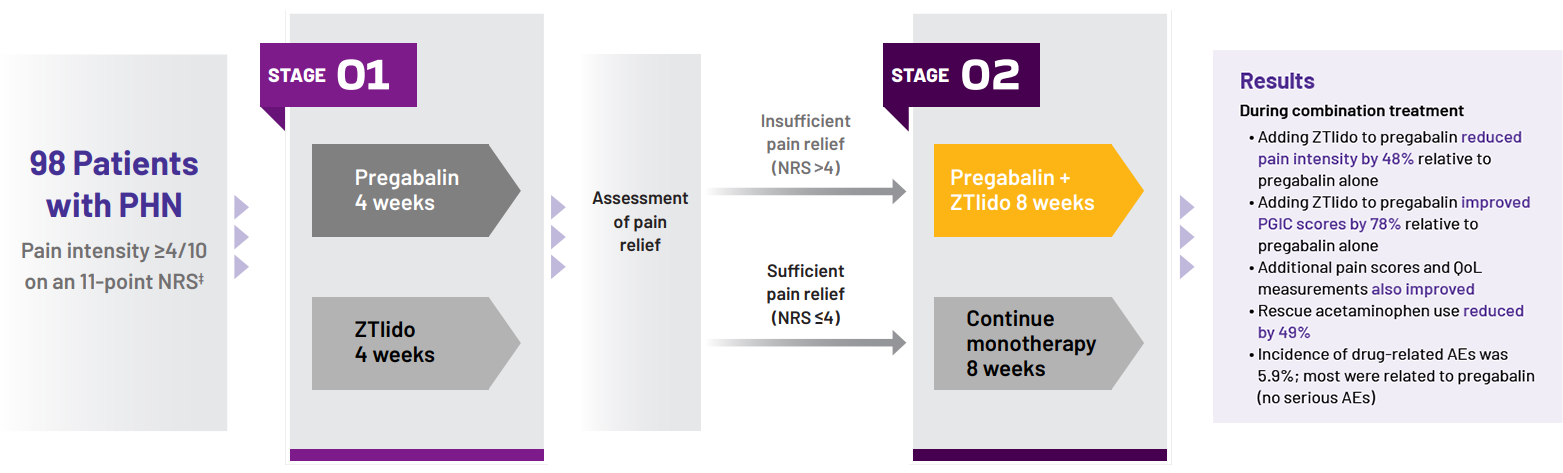
Stage 1:
Patients randomized to 4 weeks’ monotherapy with either
- Pregabalin titrated to effect
- All patients received 150 mg/day in week 1 and 300 mg/day in week 2
- At the end of week 2, dose increased to 600 mg/day in
patients with insufficient relief (NRS >4)
- ZTlido: up to 3 patches/day (12 hours on, 12 hours off)
STAGE 2:
- Sufficiently treated with monotherapy (NRS ≤4), continued the same agent for 8 weeks
- Insufficiently treated with monotherapy (NRS >4), received both drugs in combination for 8 weeks
- Results presented from patients insufficiently treated with pregabalin monotherapy
*ZTlido equivalent; "ZTlido equivalent" connotes that study was performed using bioequivalent lidocaine 5% patch.
†Concomitant analgesics not permitted, except rescue acetaminophen (≤2 g/day).
‡11-point NRS (0=no pain to 10=pain as bad as you can imagine).
*SF-MPQ pain intensity was assessed on a VAS of 0 (no pain) to 100 (worst possible pain).
†ZTlido equivalent.
"ZTlido equivalent" connotes that study was performed using bioequivalent lidocaine 5% patch.
IMPORTANT SAFETY INFORMATION
Side effects of ZTlido include application site reactions such as irritation, erythema, and pruritus. These are not all of the adverse reactions that may occur.
TESTIMONIAL
For an optimal viewing experience, please ensure the video sound is turned on.
Hear what your peers are saying about ZTlido
Ensure adequate coverage of the painful area

Use daily for at least 8 weeks

To get the full therapeutic effect, ZTlido must be used as prescribed every day (not PRN) for at least 8 weeks.
Do not allow substitutions

ZTlido is not AB-rated/ interchangeable with other lidocaine patches.11
Provide co-pay card and track progress